 |
Marsico
Hall Microscopy
Fellowship
(MHMF.ORG) |
 |
|
Leica SP2
Laser Scanning Confocal Microscope
|
|
Location: 6123 Thurston Bowles
|



Index
0. Notices - New computer installed
September 25, 2013
1. Operating the system
(a
step by step guide to basic operation of the confocal)
2. The System
3. First time use (new user)
4. Known Bugs
5.
Viewing Leica Files - getting viewing
software for Windows
6. Leica confocal file analysis and processing
7. Links
8. Reporting Problems
9. Password problems/German key board
10. Dealing with software crashes and microscope stand lockups
0.
Notices
- A new computer was installed on Sep 24, 2013. Most
functions will work as they did on the old computer. Some points worth noting are:
- Please let us know if you would like assistance or have
questions or find anything odd
- During LCS initialization (while the drosophila embryo image is
rotating) the process will stop at initializing at "Fluorescence
Module". To continue move the mouse or press shift to proceed.
- Default data directory is
D:\USERS\your-login-name
(which should contain a LCS subdirectory - see/contact Michael
if a LCS directory is not there for your directory)
- Due SOM firewall D:\Users
is no longer shared as
\\Laplace\users1 on the
network. Store data at \\minsky
- New/modified/added files at the
D:\Users
directory are copied to the main file server
\\Minsky\laplace\users1
every evening at about 2 AM.
- USB ports are available on the computer and may be used to
transfer data. Windows
XP does not support USB 3, so transfers will be at USB 2.0
speeds. Do not use a USB drive
if you are not absolutely sure that it is free of viruses, trojans and malware.
- The display screens may look brighter due a new video card. This has no effect on the acquired images.
Hopefully you are using the glow over & under scale while
imaging to set gains and offsets. Use the Q-Lut button.
- Be very careful,
deliberate and mindful around the computer and the objectives.
- Also worth noting:
- Mappings, (M: drive) to the file server \\Minsky will need
to be hand recreated for each users (ask Michael for help or
instructions)
- Passwords can be changed at the \\Laplace computer (or an
MHmicroscopy computer in room 6129 or 6026)
- Image files will need to be transferred to \\Minsky
for network access from your lab/office.
- Old Data:
- Files older than June 2012 and a few more years back (may be
up to ~4 years ago) are on the backup servers and can be made available by
arrangement with Michael or Neal
- General:
- Please help us out and let us know if you notice any quirks
on the system
- Please let us know if you would like assistance or have
questions
|
- Changing Passwords:
- Post Clean Install Notes - same as:
- System Crashes & Recovery
- There are multiple CPUs in the scan head, microscope stand
and main PC, which can individually crash causing the system to lockup in
various ways. The quickest route to recovery is to determined which
subsystem has locked up.
- Microscope stand freezes - e.g. focus drive and filter
shutter or changer does not respond: Shut down LCS software on PC,
power down stand (controller [2]), wait 15s, restart stand, then the LCS software
- LCS software can not initialize the hardware - window
with yellow diagonal line appears on computer screen: Check that
objective turret rotation is in a click position, shut down LCS software and
restart it.
- Scanning freezes or scanning can not be stopped - shut down LCS
software,
power down Scan electronics ("scanner" [5]), power down microscope stand
(controller [2]), restart stand,
restart scan electronics, run LCS software
- If the above fails - shutdown LCS software, turn off
scan electronics ("scanner" [5]), turn off microscope stand (controller [2]), if any error involves the SCSI
system, shut down Windows and power off the PC computer, but leave lasers
running. Restart the system following the standard start up
procedures.
- Please make a brief note of what happened in the log book
for each crash! This helps us keep appraised of the system's health.
- More words on dealing with crashes and lock ups.
-
 Erratic
movement of the motorized stage
Erratic
movement of the motorized stage
- On very rare occasions the motorized stage will move
up unexpectedly if the objective turret is turned, even slightly.
In order to avoid injury when cleaning immersion fluids off the
objective used, move it to two positions to the right (or left) away
from the optical path
- Lower stage down using coarse focus button on
right side of microscope stand
- Remove slide
- Turn objective to be cleaned from the light path
to two positions to the right (or left)
- Gently clean objective with lens tissue.
Blot on the glass front element. Rub slowly on the metal
around the glass element.
- Please sign the log book in the room and note any problems.
-
 Switching to dual monitor mode if single screen mode
comes up
Switching to dual monitor mode if single screen mode
comes up
- Due termination of the service contract Leica support is not longer
available at 1-866-830-0735.
- Here is the
SP2
TCS software manual
(10 MBytes)
- (An MHmicroscopy account may be required for access. Remember to put
mhmicroscopy\
before your user name)
- Upper Wallaston prism (objective Wallaston) will exacerbate
reflections during scanning especially with dry (non immersion) objectives.
Switch
objective prism wheel to bright field position BF when
not doing DIC/Nomarski.
- Transmitted light scanning through the condenser can only be done with
visible laser illumination. It can not be performed
with the UV excitation laser alone. Turn on a visible laser even if
the wavelength is not required for fluorescence.
-
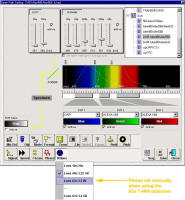 For
DAPI scanning please make sure the UV correction lens is set to the 63X.
Note that it will set automatically for the 40X 1.25 NA and 10x/20x
objectives, but needs manual intervention when the 63x 1.4 NA objective is
selected. This is a work around.
For
DAPI scanning please make sure the UV correction lens is set to the 63X.
Note that it will set automatically for the 40X 1.25 NA and 10x/20x
objectives, but needs manual intervention when the 63x 1.4 NA objective is
selected. This is a work around.
1. Operating the system
 Power up, setting up microscope,
starting software, scanning, saving, shutting down, special scanning modes
Power up, setting up microscope,
starting software, scanning, saving, shutting down, special scanning modes
2. The System
- Laser Spot Scanning Confocal Microscope
- Upright microscope with DIC (Nomarski and epi-fluorescence) DM-RXA2
- Visible (blue, green & red) and UV excitation lasers switched/modulated using an AOTF
- Simultaneous transmitted light or DIC imaging while scanning confocally
- AOBS for splitting of excitation lines from emission bands
- Photo Multipliers Tube (PMT) light detectors with spectral
discrimination
- High precision galvanometer z-axis positioning stage
2.1 Lasers:
- "UV" 351/364nm for e.g. DAPI, Hoechts,
Alexa 350, Kaeda
photoactivation, Indo-1
- "Blue" Argon 458/476/488/514nm
e.g. 488
nm FITC, Bodipy, Alexa 488. GFP, CY2, Fluo-4, Calcein; 458 nm CFP,
514 nm YFP
- "Green" Solid State Diode Pump 561nm
e.g. Texas
Red, CY3, Alexa 568, Alexa 594, will excite Rhodamine, TRITC, DsRed & Alexa 543 -
"Red" HeNe 633nm e.g. CY5, Alexa 633, Alexa 647,
TO-PRO-3, Draq-5
2.2 Objectives:
| Objectives * |
| Turret position |
Mag. |
NA |
type |
WD |
corrections |
cover slip |
Immersion |
part no. |
For DIC (Nomarski) |
| Objective Wollaston *** |
Condenser Wollaston |
| |
10x |
0.4 |
|
3.6 mm |
|
#1.5 |
air |
|
|
|
| |
16x |
0.5 |
PL Fluotar |
150 um |
|
#1.5 |
oil/glycerol/water |
506012 |
C |
2 |
| |
20x |
0.7 |
PL Apo |
590 um |
|
#1.5 |
air |
506513 |
C |
2 |
| In drawer |
40x |
0.85 |
PL Apo |
240 um |
corr |
0.14-0.18 |
water |
|
|
|
| |
40x |
1.25 to 0.75 |
Apochromat |
240 um |
aperture |
#1.5 |
oil |
|
E |
5 |
| |
63x |
1.4 to 0.6 |
PlanApo |
90 um |
aperture |
#1.5 |
oil |
|
E |
4 |
| In drawer |
63x |
1.2 |
Apo |
220 um |
corr |
0.14-0.18 |
water |
|
D |
5 |
| In drawer |
L40x |
0.8 |
HCX Apo |
3 mm |
U-V-I |
none |
water
** |
506155 |
C |
3 |
| In drawer |
L63x |
0.9 |
HCX Apo |
2 mm |
U-V-I |
none |
water
** |
506148 |
|
|
* Note: exact objective installed should be
confirmed by looking at the microscope
** dipping lenses use
no cover slip. All other lenses prefer a number 1.5 (170 um) glass cover
slip.
*** When not using DIC
the objective Wollaston should be in the blank (BF) position and the Analyser
should be out
Use only Leica immersion oil
*Counter-clockwise= increasing position number
↑
clockwise= decreasing position number
↓
2.3 Detection:
- Filtering through an Acoustical Optical Beam Splitter (AOBS)
- Spectral separation through a prism
- Three tunable detection bands with adjustable spectral windows before
the three Photo Multiplier Tubes (PMT)
- Standard XY scanning with zoom and rotation (N.B.
image rotation is not available with UV excitation)
- XZ rapid scanning using galvanometer stage (range +/-85 um)
- XZ scanning using microscope stand's fine focus (range mm's)
- Transmitted light and DIC (non confocal)
- FRAP, Emission Spectra, ratio imaging
2.4 Analysis:
- Intensity profiles, FRAP, FRET, fluorograms, 3D renders, maximum projection, average
projection
|
Epifluorescent cubes used for viewing by eye
(not confocal scanning)
|
|
LCD display |
Carousel Position # |
Leica cube ID |
Leica part |
Excitation Color of light |
Emission Color |
Typical fluorophores |
|
|
1 |
(Empty) |
- |
- |
- |
- |
|
|
2 |
(Empty) |
- |
- |
- |
- |
|
A |
3 |
A |
513824 |
Light blue BP360/40 |
Blue LP425 |
DAPI |
|
N21 |
4 |
N2.1 |
513832 |
Green BP539/45 |
Red LP590 |
Texas Red/Rhodamine/CY3 |
|
Displays I3 |
5 |
L5 |
513849 |
Blue
BP480/40 |
Green BP527/30 |
FITC, GFP, CY2 |
|
Displays L5 |
6 |
I3 |
513828 |
Blue BP470/40 |
Yellow LP515 |
YFP, FM 1-43 |
|
(SCAN) |
7 |
(Empty) |
- |
- |
- |
-s |
|
JST |
8 |
(unlabeled) |
- |
(A 3% reflective mirror cube for alignment of arc lamp) |
Carousel counter-clockwise= increasing position numbers ▲
Carousel clockwise = decreasing position numbers ▼
Leica fluorescent cube information
3. First time use (new user)
Copy LCS parameter files from d:\users\_typical\lcs to d:\users\username\lcs
Do not use factory default settings since most parameters are not appropriate
for our system
4. Known Bugs
- Parameter window zoom factor and several
other factors does not
update when loading an old image. To see these parameters, just right
click on the image name in the experiment window to get a table of scan
information. This information can also be gleaned from the .txt file
in the image database directory.
5. Viewing Files
- LAS-AF lite v 2.6
Leica LCS Light v 2.6 (Looks and somewhat runs like the LCS software on
the confocal \\Laplace computer)
- This program runs under WindowsNT4/WindowsXP
- It is available from
Users' Web pages
(http://malus.med.unc.edu/).
- Note that LeicaLCS lite does not run under Windows2000, Vista or Win 7/8
(Use LAS AF lite instead)
- A simple guide with an example image file on using Leica LCS lite
- Volocity 3D/4D image
viewing, process & measurement will import Leica image data bases
and run Mac or Windows
- ImageJ
- There are several plug ins for Leica image stacks/databases
- LOCI ImageJ plug ins
- FIJI ImageJ plug ins
Note: Read/Write permission may be required required to open a Leica Image
Database: Image data base files must be in a location with read & write
permissions. Since CD/DVDs are read only, copy the whole database to
the hard drive, and open it from the hard drive if necessary.
Depending on the operating system permissions may need resetting on the hard
drive after copying from CD/DVDs.
8. Leica confocal Image analysis and processing
7. Links
www.leica-microsystems.com
LeicaLCS-lite
software FTP site - ftp://ftp.llt.de
8. Reporting Problems
Please contact facility
personnel
Leica Confocal Service and Support
866-830-0735 8:00 A.M. to 5:00 P.M.
EST/EDT
confocal@leica-microsystems.com
serial number 195353
Note: if you contact Leica
directly please inform facility personnel as well so that we can follow up.
Installation date: 23 April 2002
9. German Keyboard mappings can
prevent passwords from being recognized
German keyboard mappings translates
some keyboard keys to other characters. Basically some keys have a
different meaning to the label embossed into the key cap. Characters to
avoid include: ! @ # ' there are others.
These characters may be found in other unusual positions on the keyboard.
10. Dealing with software crashes and Microscope
Stand Lock Ups
11. Applications:
|
 |
Last Updated:
2015-12-28 |
 Erratic
movement of the motorized stage
Erratic
movement of the motorized stage Switching to dual monitor mode if single screen mode
comes up
Switching to dual monitor mode if single screen mode
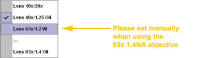
comes up For
DAPI scanning please make sure the UV correction lens is set to the 63X.
Note that it will set automatically for the 40X 1.25 NA and 10x/20x
objectives, but needs manual intervention when the 63x 1.4 NA objective is
selected. This is a work around.
For
DAPI scanning please make sure the UV correction lens is set to the 63X.
Note that it will set automatically for the 40X 1.25 NA and 10x/20x
objectives, but needs manual intervention when the 63x 1.4 NA objective is
selected. This is a work around.
